CHOARY
3.1.1 Bandgap 본문
3.1.1 Bandgap
The fundamental difference between a semiconductor and an insulator or a
conductor is the bandgap. Atoms form all materials, and every atom has its own
orbital structure [see Fig. 3.2(a)]. Electron orbits of the atom are called shells
because electrons orbit around the nucleus in a 3D shell. Thus, the orbits in
Fig. 3.2(a) can be seen as the cross section of these shells. The outermost shell
is called a valence shell.
반도체와 절연체의 기본적인 차이는 밴드갭이다
모든 물질은 원자로 이루어져있고
모든 원자는 각각의 오비털 구조를 가진다.

원자의 전자 오비탈은 shell이라고 불린다.
가장 바깥쪽은 valence shell이라고 불림
Electrons in a valence shell cannot conduct an electric current. When an electron escapes the constriction of the nucleus and leaves the
valence shell, it becomes a free electron and can conduct an electric current.
When many atoms bond together to make solid materials, their orbits overlap
and form bands, as shown in Fig. 3.2(b). Electrons in conducting bands can move
relatively freely inside solid materials and can conduct electric currents when an
electric field is applied to the solid material. Electrons in the valence band are
bound with the nuclei and cannot move freely; therefore, they cannot conduct
electric currents. Since the valence band has lower electric potential, electrons
always tend to stay in the valence band.
Resistivity is the ability of a material to resist an electric current. A good
conductor has a very low resistivity, and a good insulator (or dielectric) has a
very high resistivity. The most commonly used unit of resistivity is micro-ohm times centimeter (µΩ · cm). At room temperature, the resistivity of aluminum is
2.7 µΩ · cm, sodium is 4.7 µΩ · cm, intrinsic silicon is about 1011 µΩ · cm, and
silicon dioxide is >1018 µΩ · cm.
valence shell에 있는 전자는 전류를 만들어내지 못한다
핵의 속박에서 벗어나 valence shell을 벗어날때 이 전자들은 자유전자가 되고 전류를 만들어 낼 수 있음
많은 전자들이 고체를 만들어내기 위해 결합될때 이 오비탈들은 overplap 되어 밴드를 형성함

양자역학적으로 봤을때는 이 밴드갭을 이해하려면 엄청난 양을 더 공부해야한다.
반도체소자물리학에서 배운 내용 복습하쟝
conducting band들에 있는 전자들은 상대적으로 자유롭게 고체를 움직이고 전류를 만들어낸다
valence band에 있는 전자들은 당연히 못 움직임
그 밑에는 진짜 당연한 설명이라 생략함니다.. 몰라서 그러는거 아님
For most metals, the conducting band and valence band overlap or have a very
small bandgap, so small that electrons with thermal energy at room temperature
(300 K ≈ 0.0259 eV) can jump across it. One electron volt (1 eV) is the energy
gained by an electron when it passes through two points with one volt (1 V) of
voltage difference. Therefore, a conducting band has many electrons, explaining
why metals are always good electrical conductors. For dielectrics such as glass and
plastic, the bandgap is so large that electrons cannot jump across it from the valance
band, so the conducting band has very few electrons to conduct electric currents.
For semiconductors, the bandgap is somewhere between that of conductors
and insulators; for instance, silicon is 1.1 eV, germanium is 0.67 eV, and gallium
arsenate is 1.40 eV. While most electrons stay in the valance band, there are always
some thermal electrons (from the tail of the Boltzmann distribution, discussed in
Chapter 7) that can jump into the conducting band and conduct electric current.
For intrinsic silicon at room temperature (300 K), there are about 1.5 × 1010 per
cubic centimeter (cm−3
) electrons in the conducting band. This means at room
temperature, only about one in ten trillion electrons is in the conducting band,
while the majority of electrons remain in the valence band. Therefore, intrinsic
semiconductors can conduct electric currents at room temperature better than
dielectrics, but not as well as conductors.
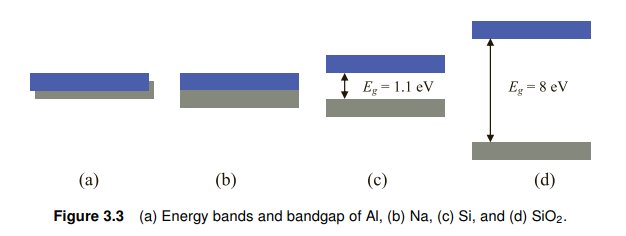
대부분의 금속들은 valence band 와 conducting band가 overlap되어있음 (A) 보이시져?
또는 (B)처럼 매우 조금 띄어져잇거나... 띄어져잇는거임 암튼 그러함
그래서 RT 에서도 쉽게 전자가 자유전자로 됨 (RT-room temperature 다 아시져?)
절연체는 밴드갭이 커서 자유전자가 되기가 어려움..
반도체는 이 금속와 절연체의 중간의 밴드갭을 가지고 있음 사실 간잽이임..
While most electrons stay in the valance band, there are always
some thermal electrons
그래서 반도체에서 대부분의 전자는 valence band에 있음.
'SEMICONDUTOR > Book summary' 카테고리의 다른 글
| Chapter 3 59P [부제: 시작이 반이다] (0) | 2021.08.01 |
|---|---|
| Chapter 2 Introduction to Integrated Circuit Fabrication (0) | 2021.03.03 |
| Introduction to Semiconductor Manufacturing Technology Chapter 1 Introduction (0) | 2021.02.17 |



